Text requirements
- INCIs for INCI relevant products (please find here a list of INCI relevant product types + a formatting orientation)
- Manufacturer specifications for foodstuff and supplements (please see here an example how we need the information)
- Marking of potential allergens for foodstuff and supplements (please see here an example how allergens can get marked)
Supplements
Product name
Needs to correspond to the official sales description of the food.
Product description
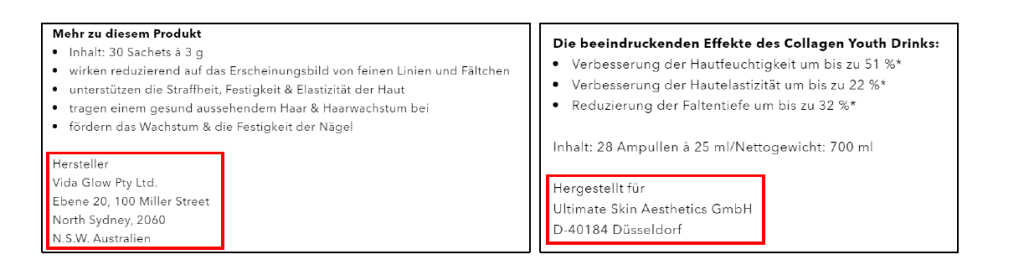
At the end of the product description, the name/company, address and country of the manufacturer or the distributor must be given:
Text how to use
According to Art. 14 of the Food Information Regulation the application text must contain the following information:
- Storage instructions
- If necessary, special information on the instructions for use
- Ingredients (INCIs)
- List of ingredients/allergens (allergen marks must be in BOLD and in CAPITAL LETTERS)
- Nutrition information
- Nutrition information must be uploaded as the last product image:
INCI
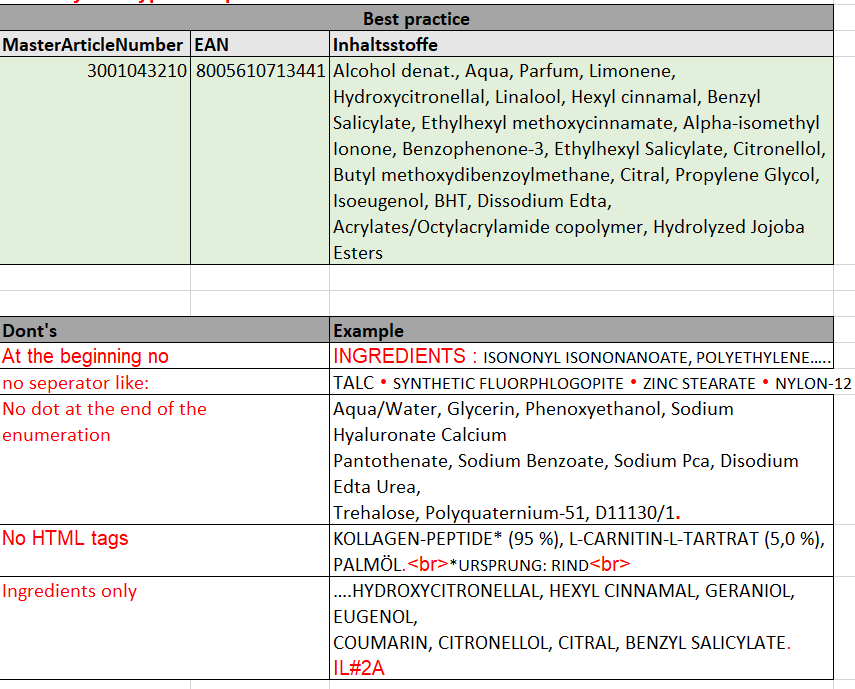
- Seperation of the ingredients only by comma (,) and no other seperation signs
- Please do not use the word “INGREDIENTS“ in front of the ingredient list
- Do not use a dot (.) at the end of the ingredient list
- No HTML tags
- Only list ingredients in the ingredient list
WEEE Nummer
- Every electronic tool, which is listed in our online shop, has to have a „WEEE-Reg.-Nr.“
- The „WEEE-Reg.-Nr.“ has to be provided by the supplier (maintained by „Stammdaten“ department)
- Example WEEE number: WEEE-Reg.-Nr.: DE12345678“
Advertising of medicinal products & medical devices
- Prohibition of statements that attribute a therapeutic effect to products that they do not have
- The false impression must not be created that success can be expected with certainty
- The false impression must not be created that prolonged use will not have a harmful effect
- The false impression must not be created that health is impaired by non-use
- It is forbidden to make untrue statements about the composition or nature of the products
- It is prohibited to advertise the prevention/relief/elimination of certain diseases
- Advertising relating to „normal illnesses“ such as colds, herpes, neurodermatitis is permitted
- The indications of the medicinal products must match the subject of the advertising
Mandatory information of medicinal products & medical devices
- The name of the medicinal product (cf. Section 4 para. 1 no. 2 HWG)
- The indications for use (cf. § 4 para. 1 no. 4 HWG)
- The warnings, insofar as they are prescribed for the labeling of the containers and outer packaging (cf. § 4 para. 1 no. 7 HWG)
- When advertising to laypersons (i.e. always in our case!), the following text must also be clearly legible (see Section 4 (3) HWG): „For risks and side effects, read the package leaflet and ask your doctor or pharmacist.”
Compliance with New EU Regulation on General Product Safety (GPSR)
the European Commission has recently introduced new obligations under the General Product Safety Regulation (EU) 2023/988 (GPSR), which mandates that specific information must be provided for all products sold online, including the obligation to display product recalls on our website. This regulation applies from 13th December 2024. To ensure compliance with the GPSR, it is crucial that all products distributed through our online platform meet the following requirements.
Required Information
In accordance with the GPSR, the following information must be made available for each product:
Manufacturer Information:
- Name, registered trade name or registered trade mark of the manufacturer
- Postal address
Responsible Person Information (if the manufacturer is not established in the European Union):
- Name of the responsible person
- Postal address
Product Identification Information:
- Information allowing the identification of the product
- A clear picture of the product
- Type of the product
- Any other relevant product identifier (e.g. model of the product, list of ingredients for cosmetic products, textile composition of textile products)
Safety Information:
- Any warnings or safety information applicable to the product
Action Required:
Please ensure that the above mentioned information is ready to be submitted from now on for the products you supply to us.
How to Submit the Information:
Please maintain or upload the above mentioned information directly to our supplier portal at BYRD. If you have any questions or need assistance, do not hesitate to contact our support inbox ecom_bmanaged@douglas.de.
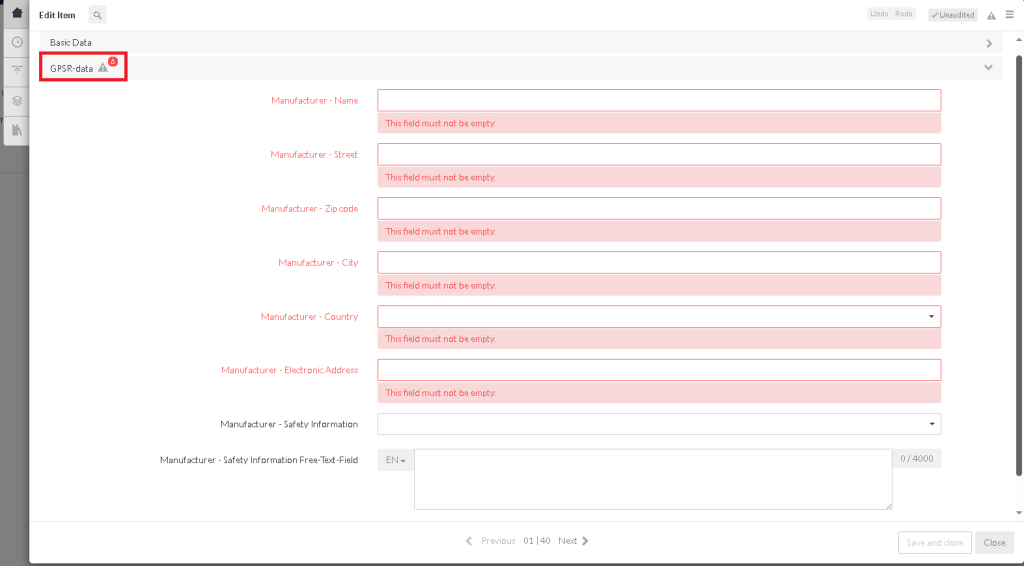
In BYRD/ bManaged you will find a new data section on your ecom item, called GPSR-data. All the fields highlighted in red are mandatory information which must be maintained.
For every already provided or existing item you must only maintain these fields and publish the item afterwards:
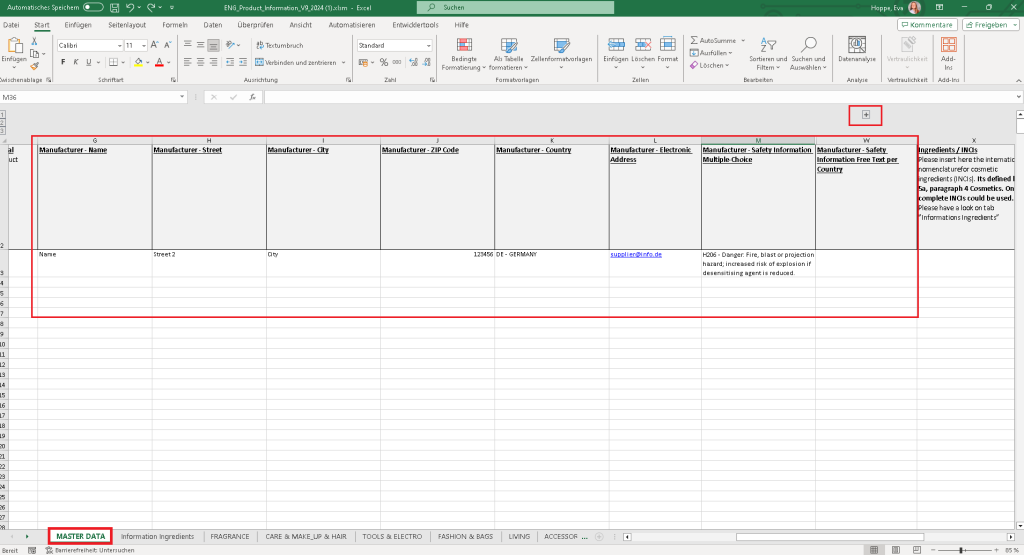
You can maintain the data via mass update, via single edit or via excel Import. You will find the respective new columns for the new fields in the MASTER DATA tab.
To enter any free text warning information, please enhance the excel file by clicking on the “+”-sign in column W. You will then have the possibility to maintain free text information per language.
After maintaining the GTIN, the needed information and after saving the file, you can simply import the file within the import section in bManaged.
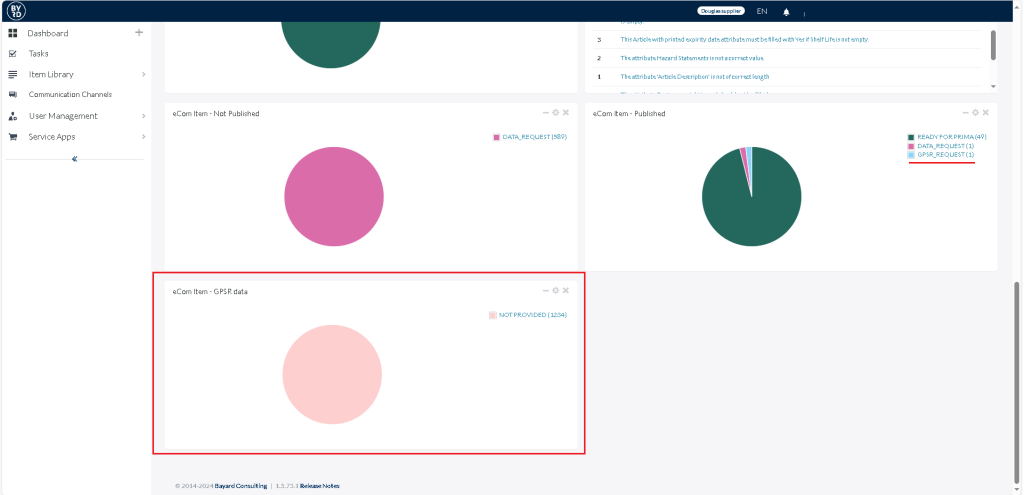
On your dashboard in bManaged you will find a new widget called “eCom Item – GPSR data”. Please provide the manufacturer information for every data set available there!
This widget shows ALL ecom items we are selling actively from you but we did not have in bManaged yet for instance because it is an older product we sell for some time already.
Additionally there is a new status called “GPSR_REQUEST” in your widget “eCom Item – Published”. Please provide the manufacturer information for every data set available there as well!
As this status is referring to items, you have already maintained in bManaged, but are not in command of the manufacturer information yet.
For every newly listed product, these manufacturer information fields are mandatory from now. If you do not maintain these fields, a publication will not be possible.
Product Recall Notification:
In the event of a product recall, you are required to notify us immediately and provide a PDF file containing all the details of the recall. You must use the template for recall notices established by the Implementing Regulation (EU) 2024/1435 of the European Commission.
This information will be displayed on our website to ensure consumer safety and compliance with the GPSR. Timely communication is essential to protect both consumers and our brand reputation.
We appreciate your prompt attention to this matter and look forward to our continued cooperation.
Thank you for your immediate action on this important issue.
